The Sidekick Electrolytes:
How Strontium, Lithium, and Rubidium Support Calcium, Magnesium, Sodium, and Potassium (and Maybe Vanadium Supports Phosphorus Too)
by Jon Sasmor RCPC (Mineral Guide, MinBalance LLC)
Updated
January 10, 2022
Summary
There are 3 Sidekick Electrolytes (strontium, lithium, and rubidium). They act at microgram, naturally occurring amounts to stabilize the well-known 4 Main Electrolytes (calcium, magnesium, sodium, and potassium).
Strontium stabilizes calcium. Lithium stabilizes both magnesium and sodium. Rubidium stabilizes potassium.
Possibly vanadium acts as an additional sidekick to stabilize phosphrous.
Alternate Summary
An hypothesis: "Sidekick Minerals" in tiny amounts act to support and calibrate the "Main Minerals", as seen on hair tissue mineral analysis (HTMA):
- Sr for Ca
- Li for Mg
- Li for Na
- Rb for K
- V for P
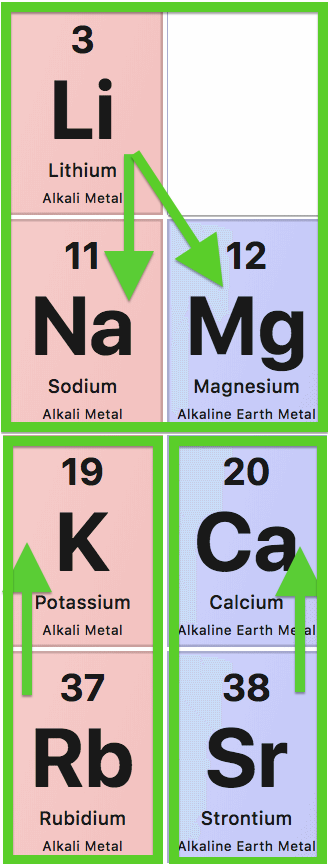
7 Electrolytes on the Periodic Table: Strontium, Calcium, Magnesium, Lithium, Sodium, Potassium, and Rubidium.
The first two columns of the Periodic Table of Elements contain (at least) 7 bioactive electrolytes: 4 "main electroytes" and 3 "sidekick electrolytes".
The sidekicks calibrate and stabilize their neighbors, as shown below:
- Lithium (Li) for both Sodium (Na) and Magnesium (Mg),
- Rubidium (Rb) for Potassium (K), and
- Strontium (Sr) for Calcium (Ca).
Excerpted from
National Center for Biotechnology Information (2021).
PubChem Periodic Table of Elements.
https://pubchem.ncbi.nlm.nih.gov/periodic-table/
.
Green markings added.
Only 4 Main Electrolytes?
Major electrolytes in the human body include calcium (Ca+2), magnesium (Mg+2), sodium (Na+), potassium (K+), phosphate (PO4-3), chloride (Cl-), and bicarbonate (HCO3-). (Shrimanker & Bhattarai 2021.)
These same electrolytes are used in intravenous electrolyte solutions, electrolyte drinks, nutrition databases, etc.
In interpretation of hair tissue mineral analysis (HTMA), we usually focus on 4 "main electrolytes" (Ca, Mg, Na, and K) and sometimes a fifth (P).
From these 4 (or 5) main electolytes, HTMA gives us great insight into the mineral patterns of individuals. This includes the methods of Dr. Paul Eck, Dr. David Watts, Dr. Rick Malter, and Dr. Lawrence Wilson.
Or Are There At Least 7 Important Electrolytes?
In addition to the 4 (or 5) Main Electrolytes, this article proposes at least 3 additional important electrolytes: strontium (Sr+2), lithium (Li+), and rubidium (Rb+), and possibly also vanadium as vanadate (VO4-3).
Dr. Schroeder's Theory of Mineral Displacement
According to Dr. Henry Schroeder's well-known mineral placeholder theory, minerals with similar properties can displace each other in enzymes in the body.
Dr. Schroeder believed that the less preferred minerals can block function of the main biologically active minerals. The substituting minerals often have similar chemical properties, such as minerals in the same column of the Periodic Table.
Some pairs of similar minerals also have synergistic interactions in which both minerals perform essential functions.
"When we go deep enough into the properties of the elements and their places and functions in living things, we cannot fail to become convinced that some of the the elements were designed for the purpose of making combinations which could live, and the way they interact is the only way in which life could exist in the Universe."
(Schroeder, 1976, p. 18. For more about Dr. Schroeder, please see Henry Schroeder: The Biological Importance of Minerals.)
Dr. Schroeder's Views of Strontium, Lithium, Rubidium, and Vanadium
Before Dr. Schroeder passed away in 1975, he wrote the following:
-
"Strontium behaves largely but not completely as an essential trace metal in the biosphere and in mammals. . . . It is possible that strontium in small amounts acts to harden the structures of bones and teeth." (Schroeder et al., 1972.)
-
Dr. Schroeder included lithium and rubidium among trace elements that "fulfill no known biological function, and are probably inert at 'normal' concentrations." (Schroeder & Nason, 1971.)
-
"[Vanadium is] possibly essential for mammals, although not yet proven so. Vanadium may have a role in cholesterol and fatty acid metabolism, depressing synthesis. Vanadium-manganese antagonisms exist in some forms of life." (Schroeder & Nason, 1971.)
Beneficial Placeholders: The Sidekick Minerals
More recent research further reveals how strontium, lithium, and rubidium (and perhaps vanadium) expand Dr. Schroeder's vision of beneficial mineral interactions.
The sidekick minerals each play a role in life's activity. They each interact beneficially with specific, similar, biologically active minerals.
How Sidekick Minerals Work
- block loss of main minerals
- signal metabolism of main minerals
- selectively promote certain functions of the main minerals relative to others
- reserve parking spaces for the main minerals, providing a buffer of extra proteins and other molecules containing the parking spaces
- each of the main electrolyte minerals has a sidekick
- at naturally occurring levels, the sidekicks synergistically support function of the main mineral
- at larger than natural (orthomolecular) dosages, the sidekicks may overpower the main mineral and block its function, making the sidekick minerals toxic at higher dosages
Specific Roles of Sidekick Electrolyte Minerals
Strontium as Sidekick to Calcium
-
Protection of Bone Calcium. Strontium has been suggested to preserve calcium in bone, both by decreasing bone resorption and by increasing bone formation, as well as by other mechanisms (Marx et al., 2020; Nielsen, 2004).
Strontium helps keep calcium in the bones, where calcium belongs.
-
Activation of Calcium Signalling. Strontium may assist calcium to trigger the extracellular calcium-sensing receptor (CaSR). Strontium may act as a partial, slightly less effective backup for calcium when bioavailable calcium levels are low; thus stabilizing calcium signalling function.
By supporting calcium to activate the CaSR at times when calcium levels are low, strontium may stabilize parathyroid activity and renal calcium excretion. Thus, strontium prevents the body from taking measures to remove calcium from bone or to accumulate excess calcium in the wrong places, inside cells.
Lithium as Sidekick to Magnesium
-
Protection of Intracellular Magnesium. Lithium has been proposed to activate fail-safe mechanisms to conserve and restore intracellular magnesium. Lithium helps keep magnesium in the cells, where magnesium belongs.
Lithium acts by mimicking low intracellular magnesium, and triggering magnesium conservation mechanisms, such as would occur in the case of traumatic brain injury. Therefore, lithium's protections from low magnesium specifically target the nervous system. (van Woerkom, 2017; see also commentary by Deans, 2018).
Today, acute stress and magnesium loss happen daily — not just in traumatic brain injury. If lithium helps our cells retain magnesium, that's hugely important!
-
Selective Inhibition of GSK-3β and Specific Other Mg-Dependent Enzymes. Lithium selectively inhibits (by filling magnesium's slot) certain magnesium-dependent enzymes, but not others. In particular, lithium inhibits glycogen synthase kinase 3β (GSK-3β), while leaving many other magnesium-dependent enzymes functioning normally.
GSK-3β likely has hundreds of substrates, fulfills numerous purposes, and affects gene expression in countless ways. GSK-3β acts especially in the mitochondria.
Inhibition of GSK-3β seems highly beneficial. Likely, GSK-3β may be overactive due to stress.
Lithium's action, by reducing GSK-3β activity:
- enhances brain-derived neurotrophic factor (BDNF) to support the nervous system
- makes glucocorticoids from stress reaction cause less stress-induced depression (better stress tolerance)
- activates AMP-activated protein kinase (AMPK), the cellular energy sensor
- inhibits mammalian target of rapamycin (mTOR)
- increases autophagy to protect tissues from injury
- restrains excess inflammation and immune activation
- enhances nuclear factor erythroid 2–related factor 2 (Nrf2) antioxidant response
These diverse effects via GSK-3β inhibition and AMPK activation may explain many of lithium's benefits.
(Jakobsson et al., 2017; Bao et al., 2019; Zhang et al., 2021).
-
ATP and GTP Binding. Lithium creates what may be a reserve supply or buffer of ATP by substituting for magnesium in complex with ATP.
Lithium-bound ATP may act comparably to fully magnesium-bound ATP, and may even prolong activation of certain ATP receptors.
Lithium-bound GTP may calm overactive G-protein cell signalling.
(Haimovich & Goldbourt, 2020; Dudev, Grauffel, et al., 2018).
Lithium as Sidekick to Sodium
-
Protection from Intracellular Sodium Buildup. Lithium enters cells via a voltage-sensitive sodium channel, and displaces sodium to protect from accumulation of intracellular sodium. Thus, lithium helps keep sodium outside the cell, where sodium belongs. Lithium acts to protect from excess intracellular sodium especially in electrically active cells such as neurons. (El-Mallakh, 2004.)
-
Stabilization of Sodium-Dependent Signalling. Lithium selectively inhibits (by filling sodium's slot) certain sodium-dependent signalling functions, but not others. Lithium inhibition may help stabilize certain specific membrane signalling receptors, when hyperactive, such as the A2AAR adenosine and the β1AR adrenergic G-protein coupled receptors (GPCRs). Lithium leaves other sodium signalling mechanisms unaffected. (Dudev, Mazmanian, et al., 2018).
Rubidium as Sidekick to Potassium
-
Less Well-Known Than the Other Sidekicks. You may not have heard of rubidium, the sidekick of potassium. Less research has been done so far, to my knowledge, about the effects of rubidium substitution for potassium, compared with the other sidekicks. This section presents indications that rubidium might turn out to be a good friend of ours.
-
Selective Substitution. Rubidium likely substitutes for potassium in some potassium functions better than others. Future research hopefully will reveal specifics.
-
Close Involvement with Na+/K+ ATPase Pump and Metabolic Rate. Rubidium closely follows potassium through the body, due to its similar characteristics.
Rubidium can replace potassium in the Na+/K+ ATPase pump, which keeps sodium outside cells and potassium inside cells. (Gill et al., 2004.)
This pump uses a significant portion of the magnesium-bound-ATP energy of the body. Thus, potassium turnover, and corresponding rubidium turnover, tracks the overall ATP metabolism.
Rubidium turnover closely tracks potassium turnover, so much so that rubidium turnover has been used as a marker to measure daily energy expenditure and metabolic rate.
(Roberts et al., 2016; Tomlinson et al., 2014).
Rubidium has been proposed to help activate the Na+/K+ ATPase pump, to keep sodium outside the cell and potassium inside the cell. Since Na+/K+ ATPase requires simultaneous binding by two external potassium ions, rubidium may aid in activation of the pump by substituting for one potassium ion. (Jenner et al., 1983.)
-
Complex Effects on Potassium Channels. Rubidium has multiple, complex effects on Ca2+-activated K+ channels:
- blocking the channel
- passing through the channel itself
- slowing the closing rate of the channel
- increasing the opening rate of the channel
-
Longer Biological Half-Life Than Potassium. Rubidium persists in the body for a longer half-life (30-60 days) than potassium (15-18 days). (Fieve et al., 1973; Jenner et al., 1983; Paschalis et al., 1978.)
Rubidium may act as a longer-lasting substitute for potassium.
Though it may fill potassium slots somewhat less effectively, rubidium persists at times of transient low potassium levels. Therefore, rubidium stabilizes potassium function.
-
Effect on Neurotransmitters. Rubidium increased norepinephrine release in rats (Stolk et al., 1970).
Rubidium enhanced serotonin (5-HT) synthesis and accumulation in rats (Wang & Grahame-Smith, 1992).
Rubidium changed the fecal microbiome of mice. Rubidium's benefits to serotonin metabolism may occur via the brain-gut-microbiota axis. (Chen et al., 2021).
-
History of Usage. Rubidium gained research interest in studies about depression. Rubidium has several opposite effects to lithium, including behavioral effects, circadian rhythms, EEG changes, and norepinephrine activation.
High-dose rubidium (much larger than nutritional amounts) experienced periods of enthusiastic popularity in the 1880s through 1910s, and again in the 1970s through 1990s. Before and after those time periods, rubidium seems mainly unknown or forgotten.
(Engelmann & Casper, 1984; Fieve et al., 1973; Jenner et al., 1983; Paschalis et al., 1978; Placidi et al., 1993; Placidi et al., 1988.)
-
Clinical Observations of Rubidium. Rubidium supplement, though little known, has been used clinically with great success.
The Bioenergy Balancing Center has made rubidium the subject of the very first in its newsletter series, and proposed several specific mechanisms for rubidium's benefits (Bioenergy Balancing Center, Rubidium, Unknown but Essential, n.d.).
Dr. Lawrence Wilson also has written enthusiastically about rubidium's attributes, benefits, and importance (Wilson, 2018).
-
Benefits to Thyroid and Cognitive Function via Supporting Potassium. One supplement manufacturer, Biotics Research, includes rubidium in certain thyroid support formulations. This suggests that rubidium boosts thyroid function.
Biotics states about its rubidium supplement: "People with poor cognitive function usually have low rubidium levels while those with good cognitive function have high rubidium levels. Use for cognitive problems in the elderly, chronic fatigue, and in difficult cases of sub-acute thyroid hypofunction." (Biotics Research NW Inc., 2015.)
On a hair tissue mineral analysis (HTMA), higher thyroid function correlates with a lower thyroid ratio (Ca/K). This means a lower calcium and/or higher potassium level.
I propose that rubidium acts to support the thyroid via increased potassium function. Rubidium on HTMA is discussed in greater detail below.
-
Rubidium in Coffee, Tea, Juice, and Bevereages. Coffee contains more rubidium (4380 mcg/L) than any other food. Rubidium may be "the secret ingredient in coffee"!
Due to its extreme water solubility, rubidium is most abundant in beverages. Tea also contains rubidium, as does juice and the cooking water from vegetables. Rubidium may be the only mineral for which coffee, tea, and other beverages deliver more than 40% of the daily intake.
More than 80% of the rubidium from carrots departs the carrots into the water during cooking. (22 mg/kg dry matter raw; 4 mg/kg dry matter cooked). Rubidium provides an important reason always to save and drink the cooking water from boiling, steaming, or pressure cooking vegetables.
Rubidium may explain the benefits many people experience from coffee enemas. Rubidium modifies the gut biome. (Chen et al., 2021). By exposure to coffee in the gut through a coffee enema, rubidium may stabilize potassium function to heal the gut.
Applications to Food and Supplements
-
More Complete Recipe for Electrolyte Nutrition. Sports drinks, intravenous (IV) electrolytes in parenteral nutrition, and all other electrolyte sources should include all the natural electrolytes, not just the main ones previously known to science.
In particular, nutritional amounts of strontium, lithium, rubidium are needed. Vanadium may be needed too.
-
Sidekick Electrolytes Replenish and Stabilize the Main Electrolytes. Sidekick Electrolytes function at smaller amounts than the Main Electrolytes (by 2-3 orders of magntitude or more). Therefore, it's easier to replenish and restore the Sidekick Electrolytes than the Main Electrolytes.
In turn, the Sidekick Electrolytes signal and support the metabolism of the Main Electrolytes. Thus, the Sidekicks, in tiny amounts, help restore, calibrate, and stabilize the levels of the Main Electrolytes.
Without the Sidekicks, huge amounts of the Main Electrolytes may be added without restoring their levels and functions. The added supplements may even destabilize the functions of the substance being added.
-
Avoid Supplementation of Calcium, Magnesium, Sodium, and/or Potassium Without Their Sidekicks. Supplementation of any of the 4 Main Electrolytes (Ca, Mg, Na, and/or K) without their Sidekicks (Sr, Li, and/or Rb) can trick the body and destabilize the Main Electrolyte mineral which is being added.
For example, consider sea salt (all the natural minerals of the ocean in balance) versus processed table salt (isolated sodium chloride).
Isolated electrolyte supplements (including table salt) can destabilize exactly those minerals which are being added!
The body expects the Sidekick Mineral in natural proportion with the Main Mineral.
If a Main Mineral is given alone without its natural Sidekick, the excess of the Main Mineral may be excreted along with a proportion of the existing supply of Sidekick Mineral.
Thus, the imbalanced supplementation further depletes the Sidekick Minerals, and destabilizes the electrolytes.
-
Harm of Agricultural Oversimplification of Nature. The Sidekick Minerals exert their benefits in plants, animals, and up and down the food chain.
Plants may grow with NPK fertilizers without Sidekick Minerals, but they won't thrive as healthily. Nor will the animals and people further up the food chain.
Nature's symphony of minerals has been replaced, in many cases, with a simpler, cheaper imitation. The effects of subtle electrolyte destabilization and imbalance tend to be passed along and accumulate intergenerationally.
Sidekick Minerals provide an example of one of many reasons to restore the soil with life and minerals, in Nature's balance. Whenever possible, eat foods from naturally raised animals and plants, grown on balanced, mineral rich soils.
For soils, farm animals, and of course, people, ocean-based trace minerals are a wonderful way to restore minerals, including the Sidekick Electrolytes.
-
Choose Natural Sources of Electrolytes Whenever Possible. Natural sources intrinsically include the Sidekick Electrolytes in natural proportions.
For those drinking adrenal cocktails, choose a natural source from coconut water or fresh carrot juice if possible, rather than from powdered potassium supplements such as cream of tartar.
For those who prefer the convenience of powdered adrenal cocktail mix, I'm investigating a possible future recipe for adrenal cocktails with added microgram amounts of strontium chloride, lithium chloride, and/or rubidium chloride. The recipes likely will need to be different for fast and slow oxidizers, and may be best if personalized based on HTMA. More details in the future.
-
Each Main Electrolyte Needs Its Sidekick!
-
When isolated calcium supplements don't seem to work, such as continued or worsened bone deterioration, it may be for lack of strontium in its natural ratio to calcium.
-
When isolated magensium supplements don't seem to work well, such as producing adrenal weakness, natural lithium may be needed to utilize the magnesium correctly (and keep it away from GSK-3β).
-
When isolated sodium in table salt causes disturbances to the body, natural lithium may be needed alongside to stabilize sodium.
-
When isolated potassium supplements don't seem to work or even worsen low-potassium symptoms, natural rubidium may be needed to stabilize potassium metabolism.
-
-
The Importance of Choosing the Right Electrolytes, Including the Sidekicks. Electrolytes aren't regulated in the body nearly so perfectly as we've been told. This means adding more of the right ones makes a big difference, when the body can't keep up with retaining them or putting them in the right place.
Electrolytes, even if not remaining in circulation very long, occupy important places in enzymes and many other molecules, where they may be stored and utilized.
The Sidekick Electrolytes are needed to help manage and regulate the Main Electrolytes. That's why we need them!
How Fluoride (in Drinking Water, Dental Products, and Dental Treatments) Insidiously Destabilizes Electrolytes
Fluoride chelates the Sidekick Electrolytes out of the body, wrecking electrolyte calibration and balance, over the course of years to decades.
Fluoride (F) is the most electronegative element; whereas strontium (Sr), lithium (Li), and rubidium (Rb) rank among the most electropositive minerals. Thus, fluoride scavenges these important Sidekick Minerals. Fluoride attracts the Sidekick Electrolytes by an unknown mechanism and chelates the Sidekick Electrolytes out of the body, perhaps through the urine.
The Main Electrolytes are electropositive too. But they're much more abundant than the Sidekicks, so fluoride (F) itself doesn't make as much of a dent in them. However, by chelating out the Sidekicks (Rb, Li, Sr), fluoride forces the body to recalibrate electrolytes at a lower level.
Fluoride may attract the scarce electropositive electrolytes, the Sidekicks, directly. Or the loss of the Sidekicks may occur by subtle kidney and/or adrenal damage that occurs from the fluoride, causing more of the Sidekick Electrolytes to leak out through the urine. In the long run, deficiency of the Sidekick Electrolytes results, which then leads to dysregulation of all the electrolytes.
Fluoride poisons us, slowly, subtly, by the chelation and depletion of the beneficial Sidekick Electrolytes. We lose the beneficial effects of strontium, lithium, and rubidium when we drink fluoridated water, brush with fluoride toothpaste, and/or get fluoride dental treatments. This, however, doesn't occur, when fluoride occurs naturally in proportion with the Sidekick Electrolytes, such as in low amounts in sea water.
Thanks to Fred Davis for suggesting how fluoride depletes lithium, due to their contrasting electronegatvity. The same problem seems likely to occur with fluoride depleting strontium and rubidium by a similar mechanism, chelating these important Sidekick / Calibrator Minerals out of the body.
Toxicity of Isolated Electrolyte Supplements and Table Salt
- Calcium and strontium are needed together, in natural proportions.
- Magnesium, lithium, and sodium are needed together, in natural proportions.
- Potassium and rubidium are needed together, in natural proportions.
Slowly and insidiously, synthetic mineral supplements confuse the body. We're not meant to encounter potassium without rubidium, nor calcium without strontium, nor sodium (table salt) without lithium and magnesium, and so forth.
Just a tiny bit of the Sidekick Minerals may be all that's needed for the body, to accompany the main minerals. And a tiny dose of the Sidekicks can help restore balance.
Natural Foods and Supplements of Electrolytes
-
Strontium (Sr) and Calcium (Ca):
- Grass-fed bone meal powder such as Living Bone by Ancestral Supplements
- Adrenal cocktails made from coconut water or carrot juice
- Bone and Ocean recipe
- Strontium chloride (SrCl2) drops by Good State (1.3mg per drop)
- Certain drinking waters provide significant strontium, "the largest increment, compared to the intake from food, of any other bulk or trace element found in water" (Schroeder et al., 1972.)
-
Magnesium (Mg):
- Ocean-sourced trace mineral drops (Aussie Trace Minerals [USA/CAN] or Amena's Daily Boost[AUS])
- Bone and Ocean recipe
- Many natural foods
-
Lithium (Li):
- Li-Zyme (50 mcg) or Li-Zyme Forte (150 mcg) by Biotics Research, from sprouted non-soy legumes
- Ocean-sourced trace mineral drops (Aussie Trace Minerals [USA/CAN] or Amena's Daily Boost[AUS])
- Lithium chloride (LiCl) drops by Good State and other brands (50 mcg per drop)
-
Sodium (Na):
- Sea salt without chemical additives, such as Hawaiian Bamboo Jade Sea Salt
-
Potassium (K) and Rubidium (Rb):
- Adrenal cocktails made from coconut water or carrot juice
- Cooking water from pressure-cooking, steaming, or boiling vegetables
- Ocean-sourced trace mineral drops (Aussie Trace Minerals [USA/CAN] or Amena's Daily Boost[AUS])
- Rb-Zyme rubidium supplement (100 mcg) by Biotics Research, from sprouted non-soy legumes
- Rubidium chloride (RbCl) drops by Good State (500 mcg per drop)
- Coffee (richest food source of rubidium) (Anke & Angelow, 1995)
- Many natural whole foods especially plant foods
-
Phosphorus (P):
- Grass-fed bone meal powder such as Living Bone by Ancestral Supplements
- Many natural whole foods especially animal foods
-
Vanadium (V):
- Ocean-sourced trace mineral drops (Aussie Trace Minerals [USA/CAN] or Amena's Daily Boost[AUS])
- Grass-fed bone meal powder such as Living Bone by Ancestral Supplements
- Grass-fed beef liver
- V-Zyme vanadium supplement (20 mcg) by Biotics Research, from sprouted non-soy legumes (now discontinued; maybe Biotics will make it again in the future)
- Organic parsley
- Bone and Ocean recipe
Avoid Most Synthetic Sidekick Electrolyte Supplements
Most synthetic supplement dosages for strontium, lithium, and vanadium are MUCH too large! They may be sold in hundreds of milligrams of strontium, milligrams of lithium, or milligrams of vanadium. These are tens to hundreds of times too much!
For comparison, when lithium, rubidium, or strontium is given as a pharma drug, the dosages used are even higher yet — on the order of 1,000 times more than what I'm suggesting here as a supplement. Use of a megadose definitely isn't recommended here and must be done under a doctor's supervision.
Also, avoid synthetic forms of Sidekick Mineral supplements not listed in the section above:
- Avoid strontium citrate or ranelate or others.
- Avoid lithium orotate or aspartate or others.
- Avoid vanadium citrate or sulfate or others
Though lithium orotate is, by far, the most popular lithium supplement, please avoid this toxic form, which also usually comes in dosages much too large. Orotate, previously known as vitamin B13, is an intermediate in pyrimidine synthesis. Orotate was popularized by Dr. Hans Nieper as a mineral carrier, which he claimed was superior to natural forms of minerals at penetrating cells. However, orotate seems at least slightly toxic and prone to inducing mineral imbalances. So please treat orotate like a synthetic B vitamin from a bottle and avoid it for the same reasons as other synthetic B vitamins.
The Progressive or Endomet brand of lithium (Lithinase) contains lithium in an appropriate dosage (50 mcg) in a food-based form (pea, lentil, and millet). However, the capsules include raw rice flour as a filler, which isn't an ancestral food. This brand may work well for some people, but doesn't make it to the recommended list above due to the rice flour.
Chloride form in natural-dosage microgram amounts may be safe: I'm currently investigating usage of strontium chloride (SrCl2), lithium chloride (LiCl), and rubidium chloride (RbCl) drops by Good State brand.
Application to Hair Tissue Mineral Analysis (HTMA)
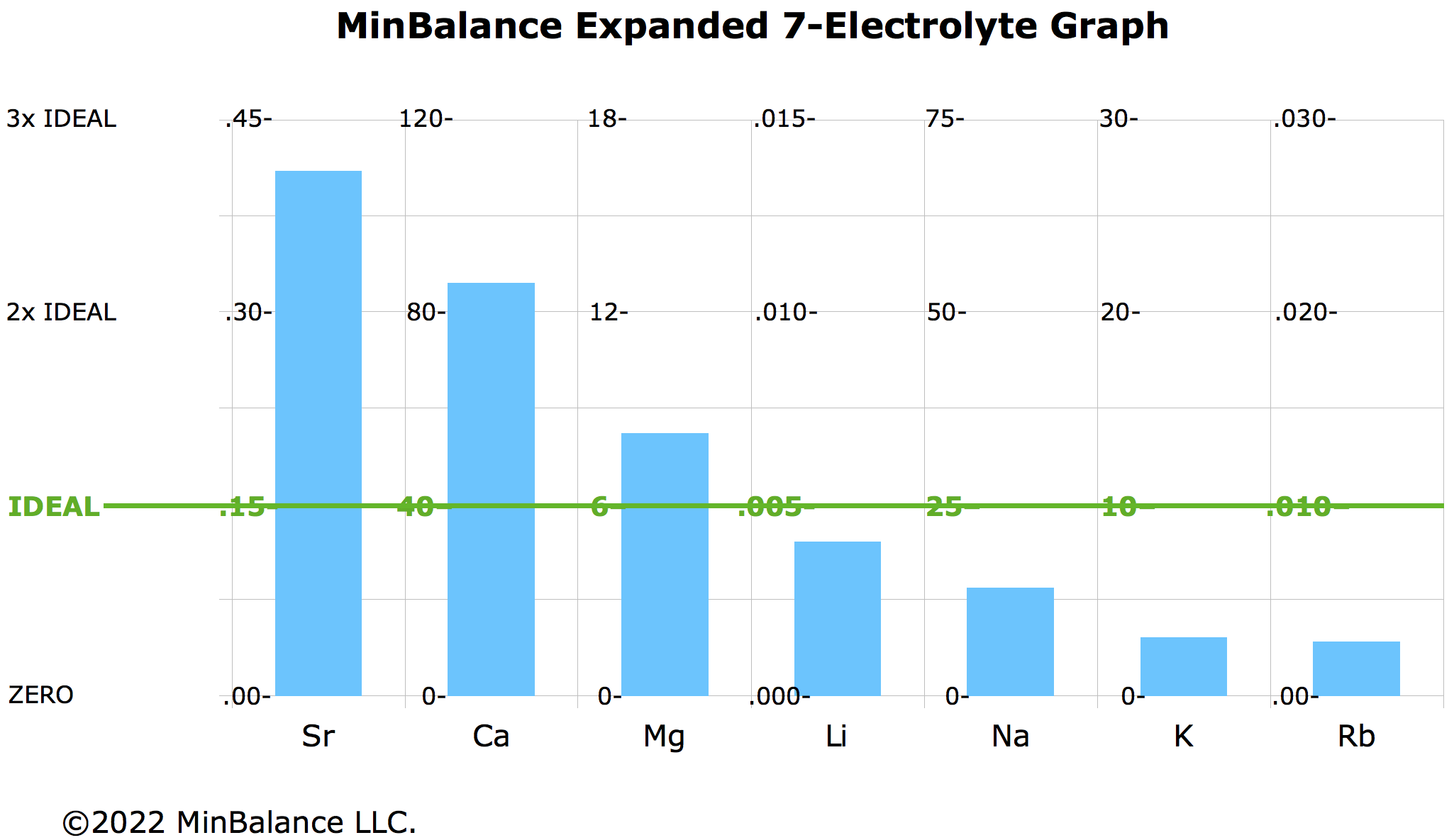
The 7-Mineral Expanded Electrolyte Graph
Dr. Eck had 4 main minerals, Ca – Mg – Na – K. To these, I've added the related minerals which stabilize each:
- Sr for Ca
- Li for both Mg & Na
- Rb for K
This makes a 7-mineral chart: Sr — Ca — Mg — Li — Na — K — Rb. The Sidekick Minerals tend to correlate with their associated Main Minerals on an HTMA.
When graphed against ideal values on a graph like below, each Calibrator / Sidekick mineral tends to be near its neighbors. This is why I call the graph "pearls on a string".
The Sidekicks are found in smaller amounts, and make more of a difference in supplements; they pull the other pearls along on the string.
Note: TEI measures rubidium, strontium, and vanadium; but ARL doesn't. Both TEI and ARL measure lithium. Other labs wash the hair in the lab before testing, which makes inaccurate results.
Like Dr. Eck and Dr. Watts, the 7-element graph follows the second column of the periodic table upward, then the first column of the periodic table downward:
7-step-down pattern on the 7-electrolyte graph. Lithium supplement likely would help, and would work better if balanced with rubidium supplement. Little if any extra strontium would be suggested.
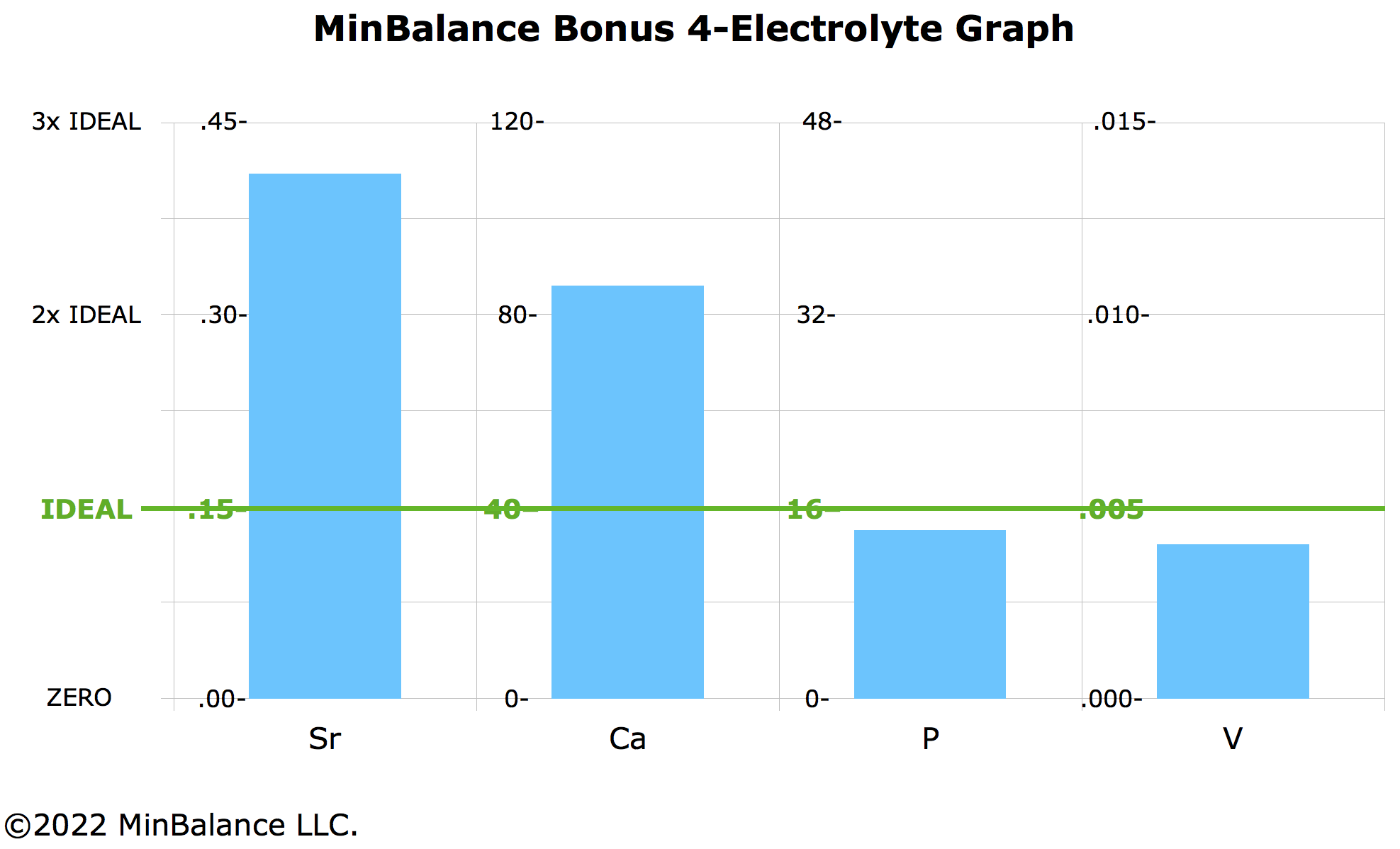
The 4-Mineral Bonus Electrolyte Graph
Dr. Watts calculates oxidation ratio with a different ratio than Dr. Eck. Dr. Watts uses Ca/P. We can make another graph for Ca and P.
- We add Sr again which stabilizes Ca.
- What stabilizes P? As an electrolyte, phosphorus is found as phosphate PO43-, which may be stabilized by... vanadate VO43-! I'm less confident about the role of vanadium compared with the other sidekicks. The reason I'm less confident about vanadium as a calibrator for phosphorus is that preliminary results seem to show less correlation of P and V, compared with the other Sidekick Minerals and their associated Main Minerals.
Then Sr — Ca — P — V might be our second important HTMA electrolyte graph:
HTMA Interpretation of Sidekick Minerals
The interpretation of the Sidekick Minerals remains an area of current research. I pay extra attention when:
- the Sidekick mineral level is absolutely high or low, or,
- the Sidekick mineral level is abnormally high or low relative to its neighbors.
HTMA Tentative Ideals for the Sidekick Minerals
- Strontium (Sr): 0.15 mg%
- Lithium (Li): 0.005 mg%
- Rubidium (Rb): 0.010 mg%
- Vanadium (V): TBD
Slow and Fast Oxidation
Dr. George Watson first identified the biotypes of fast and slow oxidation. These biotypes help determine which nutrients and foods will benefit a person. Dr. Paul Eck and Dr. David Watts pioneered the use of hair tissue mineral analysis (HTMA) to identify individuals as fast or slow oxidizers.
Imbalanced oxidation, on a HTMA, indicates imbalance between the alkali metals (Na, K, Rb) and the alkaline earth metals (Mg, Ca, Sr). Lithium serves as a bridge element between the alkali metals and alkaline earth metals due to its unique ability to substitute for both sodium and magnesium.
Usually, but not always:
-
the alkali metals (Na, K, Rb) will all tend either higher or lower than ideal. Higher usually occurs in fast oxidation. Lower usually occurs in slow oxidation or in all low electrolytes.
-
the alkaline earth metals (Mg, Ca, Sr) will all tend either higher or lower than ideal. Higher usually occurs in slow oxidation. Lower usually occurs in fast oxidation or in all low electrolytes.
-
lithium (Li) will tend to be near or between the sodium and magnesium levels. Lithium level may be the central indicator of one's electrolyte status and stability, and is shown in the center of the 7-element graph.
Effect of Sidekick Minerals on HTMA Mineral Graphs
-
Lithium raises the mineral levels from the center of the 7-element graph. Dr. Eck believed that higher overall levels, given the same ratios, indicated higher vitality.
-
Rubidium powerfully speeds oxidation rate by raising potassium function.
-
Strontium powerfully slows oxidation rate by keeping calcium in the bones, where it should be. Then excess biounavailable calcium isn't flowing into the cells.
-
Vanadium may speed the oxidation rate by raising phosphorus function. Vanadium may elevate the Na/K ratio through vanadate's inhibition of Na+/K+ ATPase pumps which restore Na/K ratio in the cell.
An Alternative Perspective: Overall Effect of Sidekick Minerals
This is an oversimplification, but may be helpful:
- Strontium slows the body and calms the mind.
- Lithium speeds the body and calms the mind.
- Rubidium speeds the body and speeds the mind.
These 3 minerals can be titrated for desired effect.
Why You Need HTMA to Assess Supplementation of Sidekick Minerals
There are some dangers of incorrect supplementation of the Sidekick Minerals:
-
Rubidium in a fast oxidizer with high K level may further raise K level and worsen symptoms.
-
Rubidium must be used with caution when Na/K is low, as it can make the situation worse.
-
Strontium in a slow oxidizer with high Ca level may further raise Ca level and worsen symtpoms. However, strontium may be needed when a calcium shell is present.
As mentioned above, the 7 electrolytes are like "pearls on a string". It may be more effective to pull on the Sidekick Minerals than the Main Minerals. This is how the Sidekicks stabilize the electrolytes.
However, the Sidekicks powerfully can pull the wrong way, if the incorrect supplement is taken. This is why we especially need a properly interpreted HTMA, plotted on the 7-element graph, to guide supplementation.
Tentative Daily Dosages for Supplementation of Calibrator Minerals
Daily dosage depends on HTMA results. These are broad, tentative ranges:
- Strontium (Sr): 50 mcg (slow oxidizer) to a few mgs (fast oxidizer)
- Lithium (Li): 50 to 500 mcg
- Rubidium (Rb): 5 mcg (fast oxidizer) to a few hundred mcgs (slow oxidizer)
- Vanadium (V): up to 5 mcg
(Note: mcg = micrograms. mg = milligrams.)
Avoid Megadoses of Sidekick Minerals
Warning: in order to obtain excellent effect from the Sidekick Minerals:
Note that the dosages at which these minerals function as powerful calibrators are MUCH smaller than those often sold as supplements (e.g. of lithium, strontium, and vanadium).
The difference is often a factor of tens or hundreds times too much in the supplements!
Lithium, rubidium, and strontium pharmaceutical dosages are even larger yet than the supplements.
The common, much larger dosages of Sidekick Minerals may have erratic results, depending on whether benefit or toxicity wins in an individual. I don't recommend supplement doses larger than those listed in the section above.
The Sidekick Minerals Jingle
Rapid Rubidium,
Slow Strontium,
Level Lithium,
Vivid Vanadium
References
- Anke, M., & Angelow, L. (1995). Rubidium in the food chain. Fresenius' Journal of Analytical Chemistry, 352(1), 236-239. https://doi.org/10.1007/BF00322334
- Bao, H., Zhang, Q., Liu, X., Song, Y., Li, X., Wang, Z., Li, C., Peng, A., & Gong, R. (2019). Lithium targeting of AMPK protects against cisplatin‐induced acute kidney injury by enhancing autophagy in renal proximal tubular epithelial cells. The FASEB Journal, 33(12), 14370-14381. https://doi.org/10.1096/fj.201901712R
- Bioenergy Balancing Center (n.d.). Rubidium, unknown but essential. Meridianlink Conference Circle. https://balancingcenter.com/rubidium-unknown-but-essential/
- Biotics Research NW Inc. (2015). Biotics Research Northwest Product Spotlight: Rb-Zyme™. https://www.bioticsnw.com/products/rb-zyme-rubidium-100t
- Chen, Q., He, Z., Zhuo, Y., Li, S., Yang, W., Hu, L., & Zhong, H. (2021). Rubidium chloride modulated the fecal microbiota community in mice. BMC Microbiology, 21(1), 1-18. https://doi.org/10.1186/s12866-021-02095-4
- Cheshmedzhieva, D., Ilieva, S., Permyakov, E. A., Permyakov, S. E., & Dudev, T. (2021). Ca2+/Sr2+ selectivity in calcium-sensing receptor (CaSR): Implications for strontium’s anti-osteoporosis effect. Biomolecules, 11(11), 1576. https://doi.org/10.3390/biom11111576
- Deans, E. (2018, December 31). Lithium: Cellular trickster. Psychology Today. https://www.psychologytoday.com/us/blog/evolutionary-psychiatry/201812/lithium-cellular-trickster
- Demo, S. D., & Yellen, G. (1992). Ion effects on gating of the Ca2+-activated K+ channel correlate with occupancy of the pore. Biophysical Journal, 61(3), 639-648. https://doi.org/10.1016/S0006-3495(92)81869-6
- Dudev, T., Grauffel, C., Hsu, S.-T. D., & Lim, C. (2018). How native and non-native cations bind and modulate the properties of GTP/ATP. Journal of Chemical Theory and Computation, 14(6), 3311-3320. https://doi.org/10.1021/acs.jctc.8b00259
- Dudev, T., Mazmanian, K., & Lim, C. (2018). Competition between Li+ and Na+ in sodium transporters and receptors: which Na+-binding sites are “therapeutic” Li+ targets?. Chemical science, 9(17), 4093-4103. https://doi.org/10.1039/C7SC05284G
- El-Mallakh, R. S. (2004). Ion homeostasis and the mechanism of action of lithium. Clinical Neuroscience Research, 4(3-4), 227–231. https://doi.org/10.1016/j.cnr.2004.09.014
- Engelmann, W., & Casper, H. (1984). Effect of RbCl on the circadian rhythm of locomotor activity in the cockroach Leucophaea maderae. Biological Rhythm Research, 15(1), 17-22. https://doi.org/10.1080/09291018409359830
- Fieve, R. R., Meltzer, H., Dunner, D. L., Levitt, M., Mendlewicz, J., & Thomas, A. (1973). Rubidium: biochemical, behavioral, and metabolic studies in humans. American Journal of Psychiatry, 130(1), 55-61. https://doi.org/10.1176/ajp.130.1.55
- Gill, S., Gill, R., Wicks, D., Despotovski, S., & Liang, D. (2004). Development of an HTS assay for Na+, K+-ATPase using nonradioactive rubidium ion uptake. Assay and Drug Development Technologies, 2(5), 535-542. (https://doi.org/10.1089/adt.2004.2.535
- Haimovich, A., & Goldbourt, A. (2020). How does the mood stabilizer lithium bind ATP, the energy currency of the cell: Insights from solid-state NMR. Biochimica et Biophysica Acta (BBA)-General Subjects, 1864(1), 129456. https://doi.org/10.1016/j.bbagen.2019.129456
- Jakobsson, E., Argüello-Miranda, O., Chiu, S.-W., Fazal, Z., Kruczek, J., Nunez-Corrales, S., Pandit, S., & Pritchet, L. (2017). Towards a unified understanding of lithium action in basic biology and its significance for applied biology. Journal of Membrane Biology, 250(6), 587-604. https://doi.org/10.1007/s00232-017-9998-2
- Jenner, F. A., Lee, C. R., Paschalis, C., Hill, S. E., Burkinshaw, L., & Jennings, G. (1983). Electrolyte metabolism in patients with periodic affective disorders during treatment with rubidium. Psychopharmacology, 81(4), 301-309. https://doi.org/10.1007/BF00427567
- Paschalis, C., Jenner, F. A., & Lee, C. R. (1978). Effects of rubidium chloride on the course of manic-depressive illness. Journal of the Royal Society of Medicine, 71(5), 343-352. https://doi.org/10.1177/014107687807100507
- Placidi, G. F., Dell'Osso, L., Nisticò, G., & Akiskal, H. S. (Eds.). (1993). Recurrent mood disorders: New perspectives in therapy. Springer-Verlag. https://doi.org/10.1007/978-3-642-76646-6 . (Includes 12 full chapters about rubidium research.)
- Placidi, G., Lenzi, A., Lazzerini, F., Dell'Osso, L., Cassano, G. B., & Akiskal, H. S. (1988). Exploration of the clinical profile of rubidium chloride in depression: a systematic open trial. Journal of clinical psychopharmacology, 8(3), 184–188. https://doi.org/10.1097/00004714-198806000-00005
- Roberts, B. R., Doecke, J. D., Rembach, A., Yévenes, L. F., Fowler, C. J., McLean, C. A., Lind, M., Volitakis, I., Masters, C. L., Bush, A. I., Hare, D. J., & the AIBL Research Group. (2016). Rubidium and potassium levels are altered in Alzheimer’s disease brain and blood but not in cerebrospinal fluid. Acta Neuropathologica Communications, 4(1), 1-8. https://doi.org/10.1186/s40478-016-0390-8
- Schroeder, H. A. (1976). Trace elements and nutrition. London: Faber and Faber. [Originally published in 1973 as The trace elements and man. Old Greenwich, CT: Devin-Adair Co.]
- Schroeder, H. A., & Nason, A. P. (1971). Trace-Element Analysis in Clinical Chemistry. Clinical Chemistry, 17(6), 461–474. https://doi.org/10.1093/clinchem/17.6.461
- Schroeder, H. A., Tipton, I. H., & Nason, A. P. (1972). Trace metals in man: Strontium and barium. Journal of Chronic Diseases, 25(9), 491–517. https://doi.org/10.1016/0021-9681(72)90150-6
- Shrimanker, I., & Bhattarai, S. (2021). Electrolytes. In StatPearls. StatPearls Publishing. https://pubmed.ncbi.nlm.nih.gov/31082167/
- Stolk, J. M., Nowack, W. J., Barchas, J. D., & Platman, S. R. (1970). Brain norepinephrine: enhanced turnover after rubidium treatment. Science, 168(3930), 501-503. https://doi.org/10.1126/science.168.3930.501
- Tomlinson, S., Mathialagan, P. D., & Maloney, S. K. (2014). Special K: Testing the potassium link between radioactive rubidium (86Rb) turnover and metabolic rate. The Journal of Experimental Biology, 217(7), 1040-1045. https://doi.org/10.1242/jeb.096222
- van Woerkom, A. E. (2017). A fully integrated new paradigm for lithium's mode of action - lithium utilizes latent cellular fail-safe mechanisms. Neuropsychiatric disease and treatment, 13, 275–302. https://doi.org/10.2147/NDT.S123612
- Wang, H., & Grahame-Smith, D. G. (1992). The effects of rubidium, caesium and quinine on 5-HT-mediated behaviour in rat and mouse—1. Rubidium. Neuropharmacology, 31(5), 413-9. https://doi.org/10.1016/0028-3908(92)90077-3
- Wilson, L. D. (2018, July). Rubidium. The Development Science And Nutritional Balancing Website. https://drlwilson.com/Articles/RUBIDIUM.htm
- Zhang, J., Anshul, F., Malhotra, D. K., Jaume, J., Dworkin, L. D., & Gong, R. (2021). Microdose lithium protects against pancreatic islet destruction and renal impairment in streptozotocin-elicited diabetes. Antioxidants, 10(1), 138. https://doi.org/10.3390/antiox10010138