Iron Deficiency? Or Iron Recycling Glitch?
by Jon Sasmor RCPC (Mineral Guide, MinBalance LLC)
Updated October 1, 2021
If you've just been told that you're "iron deficient" and that you need more iron from food and/or supplements, or that you even iron infusions, there's a catch.
95% of your iron isn't supposed to come from food, supplements, or infusions, but instead from another source! That other source is your body's efficient recycling of iron.
This article explains why you should resuscitate your iron recycling instead of adding more iron. Target 95% of your iron from efficient recycling and only 5% from new intake.
How Dietary Iron Compares with Recycling of Iron from Red Blood Cells
We begin with the following article that summarizes some widely-accepted views about iron:
Verna, G., Sila, A., Liso, M., Mastronardi, M., Chieppa, M., Cena, H., & Campiglia, P. (2021). Iron-Enriched Nutritional Supplements for the 2030 Pharmacy Shelves. Nutrients, 13(2), 378. https://doi.org/10.3390/nu13020378. Full text at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7912282/.
Verna, et al, provide a current (2021) review of the factors that promote and inhibit iron absorption. For those of us who think that hidden iron overload affects most of us, the Verna, et al, article provides a handy guide: just do the opposite of its iron-raising suggestions!
From Verna, et al:
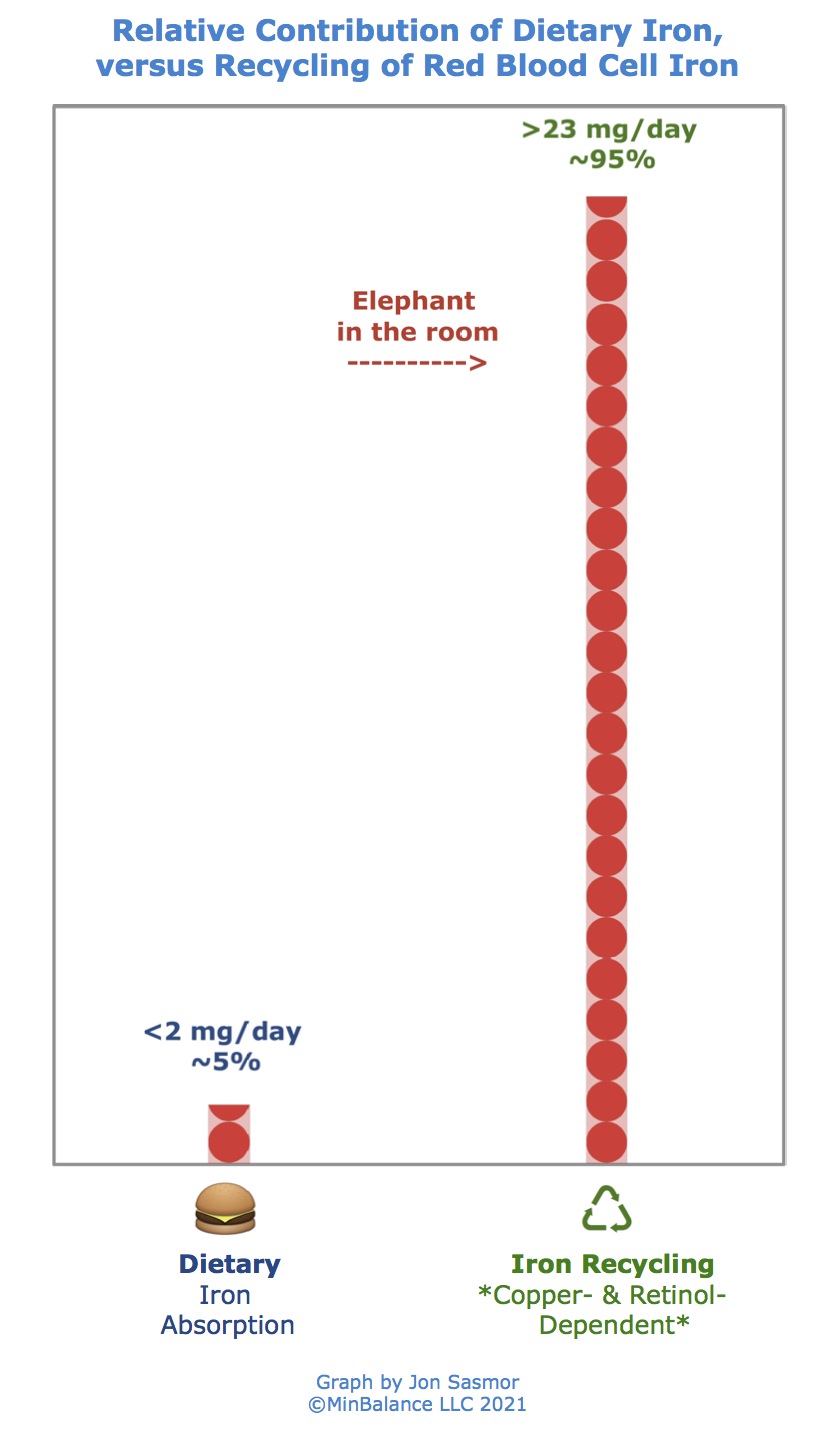
Every day, approximately 25 mg of iron are needed by our body for its correct homeostasis, most of them are required for hematopoiesis. A large part of the iron requirement is sustained by the recycling process of aged red blood cells, with only less than 2 mg still needed to be provided by food [53].
Here's a graph of how much iron we get from recycling red blood cells, versus how much iron is needed from food:
How Much Iron Do We Need to Absorb from Food?
We need to absorb from food at most, 2 mg of iron per day.
Why then do food fortication, multivitamins, and prenatals commonly provide, per serving, 18 mg or more of synthetic iron in highly absorbable forms such as FeSO4?
Verna et al seem enthusiastic about absorption of way more iron than the body's usual mechanism can accept:
Heme-iron ingested barely under 20 mg can saturate its receptor on the enterocytes and drastically reduce its absorption rate, compared to inorganic iron, which gets absorbed without any issue way over 20 mg of ingested FeSO4 (Ferrous sulfate) [59].
The question remains, if we need less than 2 mg a day, why are we giving people in excess of an order of magnitude (10x) more than what's needed?
Remember, aside from the less than 2 mg a day of iron that's lost in feces or sweat, the rest of what we need comes from recycling. It's pretty easy to replace that less than 2 mg of iron with natural whole food sources.
If There's a Shortage of Iron, It's Because Iron Recycling Isn't Working!
When I was young, my grandfather's car was leaking motor oil. Until he fixed it, he would stop every half hour or so and add more motor oil. This isn't how the engine was supposed to work! The oil was leaking, polluting, and making a mess.
Simply adding more iron with supplements, infusions, or food fortification: it's a similar approach to my grandfather adding more motor oil.
Are we making a big mess by adding extra iron, when the real issue is that iron recycling isn't working? Mineral imbalance passes intergenerationally, so the mess from adding extra iron may be getting worse each generation.
Oxidative Stress: Why to Avoid Excess Iron
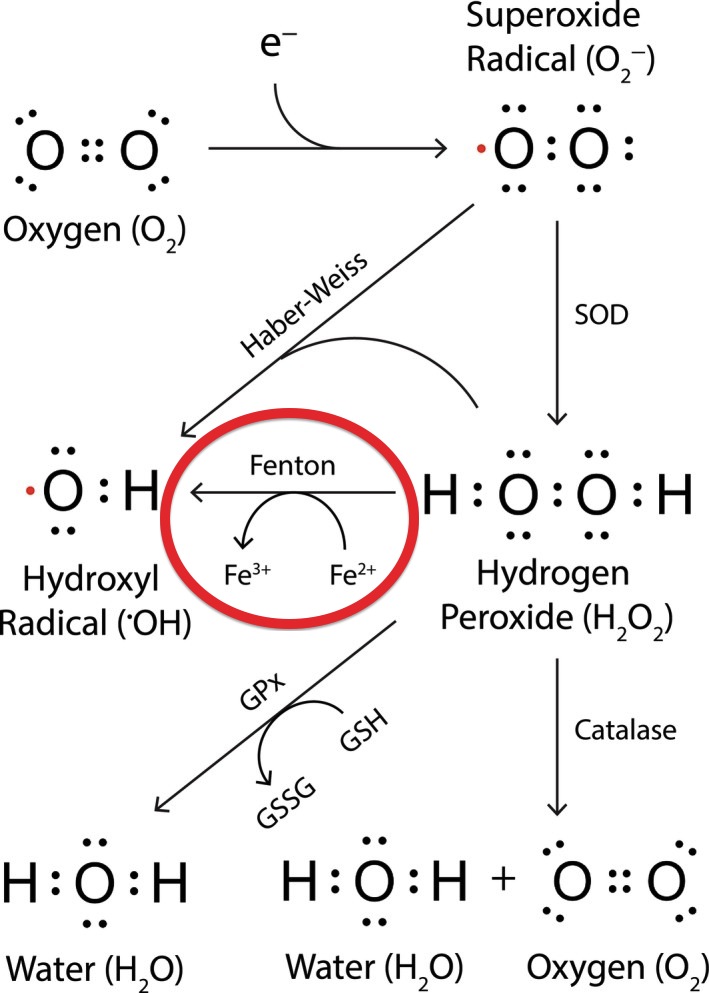
Iron is a powerful oxidant! If the iron isn't recycling, it's stuck in the cells, causing oxidative stress.
Here's a diagram of the Fenton reaction, showing how iron causes oxidative stress by creating damaging hydroxyl radicals:
Source: Trist, B.G., Hare, D.J., & Double, K.L. (2019). Oxidative stress in the aging substantia nigra and the etiology of Parkinson's disease. Aging cell, 18(6), e13031, Figure 1. https://doi.org/10.1111/acel.13031. Full text at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6826160/. Reprinted under CC-BY-4.0 Open Access license. Red circle added to emphasize excess iron's role in oxidative stress.
This is a video of what happens when iron meets hydrogen peroxide and ascorbic acid. This is the same Fenton reaction circled in red in the oxidative stress diagram above. You don't want extra iron in your cells, doing this! Please watch the 1.5-minute video to see what I mean. (Thanks to Morley Robbins for mentioning this video in Iron Toxicity Post #67!)
Are You Getting More than 10x the Iron You Need?
The required amount of iron absorption from food, less than 2 milligrams per day, seems feasible to replace from whole foods, in a variety of different ways in a variety of different natural diets. If 1 or 2 milligrams are needed, how is it ever a good thing to add synthetics an order of magnitude greater (10 or 20 milligrams or more)? That's often the amount in each serving of your prenatal, multivitamin, bread, pasta, pizza, pancakes, and breakfast cereal! And every day, day after day, you might eat 5 or 10 servings of iron-fortified food, each serving containing 10 times more iron than you need for the whole day.
If you add more iron when recycling isn't working right, then iron's getting stuck.
If we need <2 milligrams absorbed from food + >23 milligrams from recycling, and if we give 20, 40, 60, or 100 milligrams of highly absorbed synthetic iron in fortified food or supplements, then...
- How much iron gets recycled?
- Where's the iron getting stuck that should have been recycled?
- Where's the extra iron intake going?
- What's it doing there? Did you see the video of the Fenton reaction, above?
- Does iron deficiency (1-2 mg per day needed) really exist, or does iron recycling impairment (23-24 mg per day needed), which involves other nutrients such as copper and retinol-A, seem far more likely?
How Mineral Balancing Can Help Resuscitate Iron Recycling, with Copper and Retinol-A
Excess total iron in the system isn't good for you! It's better to make the existing iron work for you more efficiently, with iron recycling! Iron recycling requires other nutrients, less talked about, such as bioavailable copper and retinol-A.
You can read about the critical roles of copper and retinol-A in the blog post Low Iron Levels Don't Mean You Need More Iron.
Mineral balancing with the Root Cause Protocol can help unravel the extra iron in your body!